Ch4 Polar Or Nonpolar | It easily reacts with metal ions to result in metal sulfides. Sep 03, 2019 · a polar covalent bond is present in compounds or radicals where the two atoms are of varying electronegativities like nh3 or ch4. The only forces left to consider are london dispersion forces. Other properties of h2s are: Constituent polar and nonpolar covalent compounds
Mar 25, 2021 · to know about the polarity of the ch4 molecule, check out our detailed blog post on ch 4 polarity to find out if the molecule is polar or nonpolar. Jul 20, 2021 · so, is ch4 polar or nonpolar? H2s is a slightly polar molecule because of its bent shaped geometrical structure and the small difference between the electronegativity of hydrogen(2.2) and sulfur(2.58) that results in a non zero dipole moment. In a nonpolar molecule, electrons are always moving. It easily reacts with metal ions to result in metal sulfides.
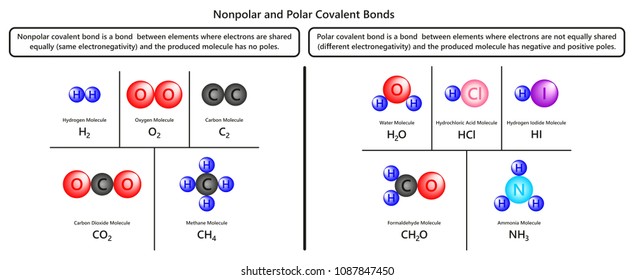
As the difference in electronegativity grows, the bond between electron pairs becomes more associated with one atom than the other atom. The charge distribution is asymmetrical and the molecule is nonpolar. The molecule would still be nonpolar. In this case, the electrons are pulled more towards one atom ( the more electronegative one) than the other. The charge distribution is symmetrical and the molecule is polar. The electronegativity of the two atoms influences how the electrons forming a bond are shared between the atoms. The only forces left to consider are london dispersion forces. Which one of the following is a polar molecule with nonpolar bonds? It is a blob with no positive or negative ends. The difference in electrostatic potential is also minimal giving an overall. Mar 25, 2021 · to know about the polarity of the ch4 molecule, check out our detailed blog post on ch 4 polarity to find out if the molecule is polar or nonpolar. Molecules that have such nonpolar bonds include ch4, n2, and h2. Which of the following ions has the same electron configuration as an argon atom?
Which one of the following is a polar molecule with nonpolar bonds? Other properties of h2s are: Which of the following ions has the same electron configuration as an argon atom? The only forces left to consider are london dispersion forces. Polar covalent bonds of chlorine, ammonia, dichloromethane, boron trifluoride and carbon tetrachloride.

Sep 03, 2019 · a polar covalent bond is present in compounds or radicals where the two atoms are of varying electronegativities like nh3 or ch4. H2s is a slightly polar molecule because of its bent shaped geometrical structure and the small difference between the electronegativity of hydrogen(2.2) and sulfur(2.58) that results in a non zero dipole moment. As the difference in electronegativity grows, the bond between electron pairs becomes more associated with one atom than the other atom. About priyanka to read, write and know something new everyday is the only way i see my day ! The difference in electrostatic potential is also minimal giving an overall. It is a blob with no positive or negative ends. The charge distribution is symmetrical and the molecule is nonpolar. In a nonpolar molecule, electrons are always moving. Jul 14, 2021 · so, is h2s polar or nonpolar? The charge distribution is symmetrical and the molecule is polar. Mar 25, 2021 · to know about the polarity of the ch4 molecule, check out our detailed blog post on ch 4 polarity to find out if the molecule is polar or nonpolar. Molecules that have such nonpolar bonds include ch4, n2, and h2. The molecule would still be nonpolar.
Which one of the following is a polar molecule with nonpolar bonds? Mar 25, 2021 · to know about the polarity of the ch4 molecule, check out our detailed blog post on ch 4 polarity to find out if the molecule is polar or nonpolar. In a nonpolar molecule, electrons are always moving. H2s is a slightly polar molecule because of its bent shaped geometrical structure and the small difference between the electronegativity of hydrogen(2.2) and sulfur(2.58) that results in a non zero dipole moment. About priyanka to read, write and know something new everyday is the only way i see my day !
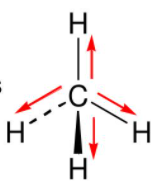
In a nonpolar molecule, electrons are always moving. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero. The only forces left to consider are london dispersion forces. Jul 14, 2021 · so, is h2s polar or nonpolar? The charge distribution is symmetrical and the molecule is polar. The molecule would still be nonpolar. The difference in electrostatic potential is also minimal giving an overall. The charge distribution is asymmetrical and the molecule is nonpolar. Which one of the following is a polar molecule with nonpolar bonds? The electronegativity of the two atoms influences how the electrons forming a bond are shared between the atoms. In this case, the electrons are pulled more towards one atom ( the more electronegative one) than the other. Which of the following ions has the same electron configuration as an argon atom? Other properties of h2s are:
Ch4 Polar Or Nonpolar: In this case, the electrons are pulled more towards one atom ( the more electronegative one) than the other.
EmoticonEmoticon